
Molecular dynamics (MD) is a
computer simulation
Computer simulation is the process of mathematical modelling, performed on a computer, which is designed to predict the behaviour of, or the outcome of, a real-world or physical system. The reliability of some mathematical models can be dete ...
method for analyzing the
physical movements of
atoms and
molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the
dynamic
Dynamics (from Greek δυναμικός ''dynamikos'' "powerful", from δύναμις ''dynamis'' "power") or dynamic may refer to:
Physics and engineering
* Dynamics (mechanics)
** Aerodynamics, the study of the motion of air
** Analytical dyna ...
"evolution" of the system. In the most common version, the
trajectories
A trajectory or flight path is the path that an object with mass in motion follows through space as a function of time. In classical mechanics, a trajectory is defined by Hamiltonian mechanics via canonical coordinates; hence, a complete traj ...
of atoms and molecules are determined by
numerically solving Newton's equations of motion for a system of interacting particles, where
forces between the particles and their
potential energies are often calculated using
interatomic potential
Interatomic potentials are mathematical functions to calculate the potential energy of a system of atoms with given positions in space.M. P. Allen and D. J. Tildesley. Computer Simulation of Liquids. Oxford University Press, Oxford, England, 198 ...
s or
molecular mechanical force fields. The method is applied mostly in
chemical physics
Chemical physics is a subdiscipline of chemistry and physics that investigates physicochemical phenomena using techniques from atomic and molecular physics and condensed matter physics; it is the branch of physics that studies chemical process ...
,
materials science, and
biophysics
Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. ...
.
Because molecular systems typically consist of a vast number of particles, it is impossible to determine the properties of such
complex systems
A complex system is a system composed of many components which may interact with each other. Examples of complex systems are Earth's global climate, organisms, the human brain, infrastructure such as power grid, transportation or communication s ...
analytically; MD simulation circumvents this problem by using
numerical methods. However, long MD simulations are mathematically
ill-conditioned
In numerical analysis, the condition number of a function measures how much the output value of the function can change for a small change in the input argument. This is used to measure how sensitive a function is to changes or errors in the input ...
, generating cumulative errors in numerical integration that can be minimized with proper selection of algorithms and parameters, but not eliminated entirely.
For systems that obey the
ergodic hypothesis, the evolution of one molecular dynamics simulation may be used to determine macroscopic
thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of the ...
properties of the system: the time averages of an ergodic system correspond to
microcanonical ensemble
In statistical mechanics, the microcanonical ensemble is a statistical ensemble that represents the possible states of a mechanical system whose total energy is exactly specified. The system is assumed to be isolated in the sense that it canno ...
averages. MD has also been termed "
statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. It does not assume or postulate any natural laws, but explains the macroscopic be ...
by numbers" and "
Laplace's vision of
Newtonian mechanics" of predicting the future by animating nature's forces and allowing insight into molecular motion on an atomic scale.
History
MD was originally developed in the early 1950s, following the earlier successes with
Monte Carlo simulations, which themselves date back to the eighteenth century, in the
Buffon's needle problem
In mathematics, Buffon's needle problem is a question first posed in the 18th century by Georges-Louis Leclerc, Comte de Buffon:
:Suppose we have a floor made of parallel strips of wood, each the same width, and we drop a needle onto the floo ...
for example, but was popularized for
statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. It does not assume or postulate any natural laws, but explains the macroscopic be ...
at
Los Alamos National Laboratory
Los Alamos National Laboratory (often shortened as Los Alamos and LANL) is one of the sixteen research and development laboratories of the United States Department of Energy (DOE), located a short distance northwest of Santa Fe, New Mexico, ...
by Rosenbluth and Metropolis in what is known today as
Metropolis–Hastings algorithm
In statistics and statistical physics, the Metropolis–Hastings algorithm is a Markov chain Monte Carlo (MCMC) method for obtaining a sequence of random samples from a probability distribution from which direct sampling is difficult. This sequ ...
. Interest in the time evolution of N-body systems dates much earlier to the seventeenth century, beginning with Newton, and continued into the following century largely with a focus on celestial mechanics and issues such as the stability of the solar system. Many of the numerical methods used today were developed during this time period, which predates the use of computers; for example, the most common integration algorithm used today, the
Verlet integration
Verlet integration () is a numerical method used to integrate Newton's equations of motion. It is frequently used to calculate trajectories of particles in molecular dynamics simulations and computer graphics. The algorithm was first used in 1791 ...
algorithm, was used as early as 1791 by
Jean Baptiste Joseph Delambre
Jean Baptiste Joseph, chevalier Delambre (19 September 1749 – 19 August 1822) was a French mathematician, astronomer, historian of astronomy, and geodesist. He was also director of the Paris Observatory, and author of well-known books on t ...
. Numerical calculations with these algorithms can be considered to be MD "by hand."
As early as 1941, integration of the many-body equations of motion was carried out with analog computers. Some undertook the labor-intensive work of modeling atomic motion by constructing physical models, e.g., using macroscopic spheres. The aim was to arrange them in such a way as to replicate the structure of a liquid and use this to examine its behavior.
J.D. Bernal
John Desmond Bernal (; 10 May 1901 – 15 September 1971) was an Irish scientist who pioneered the use of X-ray crystallography in molecular biology. He published extensively on the history of science. In addition, Bernal wrote popular book ...
said, in 1962: "''... I took a number of rubber balls and stuck them together with rods of a selection of different lengths ranging from 2.75 to 4 inches. I tried to do this in the first place as casually as possible, working in my own office, being interrupted every five minutes or so and not remembering what I had done before the interruption.''"
Following the discovery of microscopic particles and the development of computers, interest expanded beyond the proving ground of gravitational systems to the statistical properties of matter. In an attempt to understand the origin of irreversibility, Fermi proposed in 1953, and published in 1955,
[Fermi E., Pasta J., Ulam S., Los Alamos report LA-1940 (1955).] the use of
MANIAC I
__NOTOC__
The MANIAC I (''Mathematical Analyzer Numerical Integrator and Automatic Computer Model I'') was an early computer built under the direction of Nicholas Metropolis at the Los Alamos Scientific Laboratory. It was based on the von Neuma ...
, also at
Los Alamos National Laboratory
Los Alamos National Laboratory (often shortened as Los Alamos and LANL) is one of the sixteen research and development laboratories of the United States Department of Energy (DOE), located a short distance northwest of Santa Fe, New Mexico, ...
, to solve the time evolution of the equations of motion for a many-body system subject to several choices of force laws; today, this seminal work is known as the
Fermi–Pasta–Ulam–Tsingou problem
In physics, the Fermi–Pasta–Ulam–Tsingou problem or formerly the Fermi–Pasta–Ulam problem was the apparent paradox in chaos theory that many complicated enough physical systems exhibited almost exactly periodic behavior – called Fermi ...
. The time evolution of the energy from the original work is shown in the figure to the right.
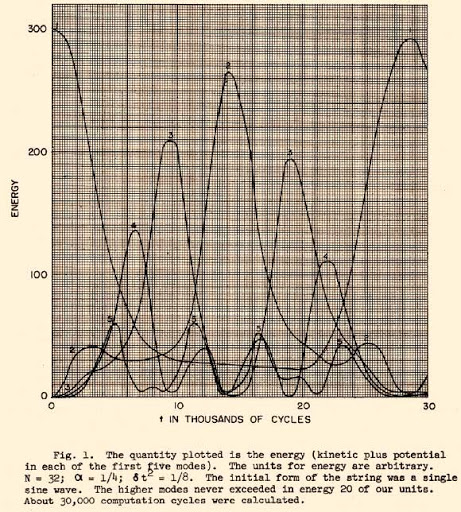
In 1957,
Alder
Alders are trees comprising the genus ''Alnus'' in the birch family Betulaceae. The genus comprises about 35 species of monoecious trees and shrubs, a few reaching a large size, distributed throughout the north temperate zone with a few sp ...
and Wainwright
used an
IBM 704 computer to simulate perfectly elastic collisions between
hard spheres
Hard spheres are widely used as model particles in the statistical mechanical theory of fluids and solids. They are defined simply as impenetrable spheres that cannot overlap in space. They mimic the extremely strong ("infinitely elastic bouncing" ...
.
In 1960, in perhaps the first realistic simulation of matter, Gibson et al. simulated radiation damage of solid
copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
by using a Born–Mayer type of repulsive interaction along with a cohesive surface force. In 1964,
Rahman published simulations of liquid
argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as ...
that used a
Lennard-Jones potential; calculations of system properties, such as the coefficient of
self-diffusion According to IUPAC definition, self-diffusion coefficient is the diffusion coefficient D_i^* of species i when the chemical potential gradient equals zero. It is linked to the diffusion coefficient D_i by the equation:
D_i^*=D_i\frac.
Here, a_i is ...
, compared well with experimental data.
Today, the
Lennard-Jones potential is still one of the most frequently used
intermolecular potentials: It is used for describing simple substances (a.k.a.
Lennard-Jonesium) for conceptual and model studies and as a building block in many force fields of real substances.
Areas of application and limits
First used in theoretical
physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which r ...
, the MD method gained popularity in
materials science soon afterward, and since the 1970s is also common in
biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
and
biophysics
Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. ...
. MD is frequently used to refine 3-dimensional structures of
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s and other
macromolecules based on experimental constraints from
X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
or
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fie ...
. In physics, MD is used to examine the dynamics of atomic-level phenomena that cannot be observed directly, such as thin-film growth and ion-subplantation, and also to examine the physical properties of
nanotechnological
Nanotechnology, also shortened to nanotech, is the use of matter on an atomic, molecular, and supramolecular scale for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal o ...
devices that have not or cannot yet be created. In biophysics and
structural biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every le ...
, the method is frequently applied to study the motions of macromolecules such as
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s and
nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s, which can be useful for interpreting the results of certain biophysical experiments and for modeling interactions with other molecules, as in
ligand docking
In the field of molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when a ligand and a target are binding (molecular), bound to each other to form a stable supramolecular chemistry, complex ...
. In principle MD can be used for
ab initio prediction of
protein structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monom ...
by simulating
folding
Fold, folding or foldable may refer to:
Arts, entertainment, and media
* ''Fold'' (album), the debut release by Australian rock band Epicure
* Fold (poker), in the game of poker, to discard one's hand and forfeit interest in the current pot
*Abov ...
of the
polypeptide chain
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
from
random coil
In polymer chemistry, a random coil is a conformation of polymers where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the ch ...
.
The results of MD simulations can be tested through comparison to experiments that measure molecular dynamics, of which a popular method is NMR spectroscopy. MD-derived structure predictions can be tested through community-wide experiments in Critical Assessment of protein Structure Prediction (
CASP
Critical Assessment of Structure Prediction (CASP), sometimes called Critical Assessment of Protein Structure Prediction, is a community-wide, worldwide experiment for protein structure prediction taking place every two years since 1994. CASP prov ...
), although the method has historically had limited success in this area.
Michael Levitt
Michael Levitt, ( he, מיכאל לויט; born 9 May 1947) is a South African-born biophysicist and a professor of structural biology at Stanford University, a position he has held since 1987. Levitt received the 2013 Nobel Prize in Chemistr ...
, who shared the
Nobel Prize
The Nobel Prizes ( ; sv, Nobelpriset ; no, Nobelprisen ) are five separate prizes that, according to Alfred Nobel's will of 1895, are awarded to "those who, during the preceding year, have conferred the greatest benefit to humankind." Alfr ...
partly for the application of MD to proteins, wrote in 1999 that CASP participants usually did not use the method due to "''... a central embarrassment of molecular mechanics, namely that energy minimization or molecular dynamics generally leads to a model that is less like the experimental structure.''"
Improvements in computational resources permitting more and longer MD trajectories, combined with modern improvements in the quality of
force field parameters, have yielded some improvements in both structure prediction and
homology model
Homology modeling, also known as comparative modeling of protein, refers to constructing an atomic-resolution model of the "''target''" protein from its amino acid sequence and an experimental three-dimensional structure of a related homologous pr ...
refinement, without reaching the point of practical utility in these areas; many identify force field parameters as a key area for further development.
MD simulation has been reported for
pharmacophore
300px, An example of a pharmacophore model.
A pharmacophore is an abstract description of molecular features that are necessary for molecular recognition of a ligand by a biological macromolecule. IUPAC defines a pharmacophore to be "an ensemble o ...
development and drug design.
For example, Pinto et al. implemented MD simulations of Bcl-Xl complexes to calculate average positions of critical amino acids involved in ligand binding.
On the other hand, Carlson et al. implemented molecular dynamics simulation to identify compounds that complement the receptor while causing minimal disruption of the conformation and flexibility of the active site. Snapshots of the protein at constant time intervals during the simulation were overlaid to identify conserved binding regions (conserved in at least three out of eleven frames) for pharmacophore development. Spyrakis et al. relied on a workflow of MD simulations, finger prints for ligands and proteins (FLAP) and linear discriminate analysis to identify best ligand– protein conformations to act as pharmacophore templates based on retrospective ROC analysis of the resulting pharmacophores. In an attempt to ameliorate structure-based drug discovery modeling, vis-à-vis the need for many modeled compounds, Hatmal et al proposed a combination of MD simulation and ligand-receptor intermolecular contacts analysis to discern critical intermolecular contacts (binding interactions) from redundant ones in a single ligand–protein complex. Critical contacts can then be converted into pharmacophore models that can be used for virtual screening.
Limits of the method are related to the parameter sets used, and to the underlying
molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
force fields. One run of an MD simulation optimizes the
potential energy
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors.
Common types of potential energy include the gravitational potentia ...
, rather than the
free energy of the protein , meaning that all
entropic contributions to
thermodynamic stability
In chemistry, chemical stability is the thermodynamic stability of a chemical system.
Thermodynamic stability occurs when a system is in its lowest energy state, or in chemical equilibrium with its environment. This may be a dynamic equilibriu ...
of protein structure are neglected, including the
conformational entropy
In chemical thermodynamics, conformational entropy is the entropy associated with the number of conformations of a molecule. The concept is most commonly applied to biological macromolecules such as proteins and RNA, but also be used for poly ...
of the polypeptide chain (the main factor that destabilizes protein structure) and
hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar ...
s (the main driving forces of protein folding). Another important factor is intramolecular
hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s,
which are not explicitly included in modern force fields, but described as Coulomb interactions of atomic point charges. This is a crude approximation because hydrogen bonds have a partially
quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
and
chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
nature. Furthermore, electrostatic interactions are usually calculated using the
dielectric constant
The relative permittivity (in older texts, dielectric constant) is the permittivity of a material expressed as a ratio with the electric permittivity of a vacuum. A dielectric is an insulating material, and the dielectric constant of an insulat ...
of
vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often dis ...
, although the surrounding aqueous solution has a much higher dielectric constant. Using the
macroscopic
The macroscopic scale is the length scale on which objects or phenomena are large enough to be visible with the naked eye, without magnifying optical instruments. It is the opposite of microscopic.
Overview
When applied to physical phenomena a ...
dielectric constant at short interatomic distances is questionable. Finally,
van der Waals interactions in MD are usually described by
Lennard-Jones potentials based on the
Fritz London
Fritz Wolfgang London (March 7, 1900 – March 30, 1954) was a German physicist and professor at Duke University. His fundamental contributions to the theories of chemical bonding and of intermolecular forces ( London dispersion forces) are today ...
theory that is only applicable in a vacuum. However, all types of van der Waals forces are ultimately of electrostatic origin and therefore depend on
dielectric properties of the environment.
The direct measurement of attraction forces between different materials (as
Hamaker constant
The Hamaker constant ''A'' can be defined for a van der Waals (vdW) body–body interaction:
:A=\pi^2C\rho_1\rho_2,
where \rho_1 and \rho_2 are the number densities of the two interacting kinds of particles, and ''C'' is the London coefficient in ...
) shows that "the interaction between hydrocarbons across water is about 10% of that across vacuum".
The environment-dependence of van der Waals forces is neglected in standard simulations, but can be included by developing polarizable force fields.
Design constraints
The design of a molecular dynamics simulation should account for the available computational power. Simulation size (''n'' = number of particles), timestep, and total time duration must be selected so that the calculation can finish within a reasonable time period. However, the simulations should be long enough to be relevant to the time scales of the natural processes being studied. To make statistically valid conclusions from the simulations, the time span simulated should match the
kinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical ki ...
of the natural process. Otherwise, it is analogous to making conclusions about how a human walks when only looking at less than one footstep. Most scientific publications about the dynamics of proteins and DNA
use data from simulations spanning nanoseconds (10
−9 s) to microseconds (10
−6 s). To obtain these simulations, several CPU-days to CPU-years are needed. Parallel algorithms allow the load to be distributed among CPUs; an example is the spatial or force decomposition algorithm.
During a classical MD simulation, the most CPU intensive task is the evaluation of the
potential
Potential generally refers to a currently unrealized ability. The term is used in a wide variety of fields, from physics to the social sciences to indicate things that are in a state where they are able to change in ways ranging from the simple r ...
as a function of the particles' internal coordinates. Within that energy evaluation, the most expensive one is the non-bonded or non-covalent part. In
Big O notation, common molecular dynamics simulations
scale by
if all pair-wise
electrostatic
Electrostatics is a branch of physics that studies electric charges at rest ( static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amb ...
and
van der Waals interactions must be accounted for explicitly. This computational cost can be reduced by employing electrostatics methods such as particle mesh
Ewald summation Ewald summation, named after Paul Peter Ewald, is a method for computing long-range interactions (e.g. electrostatic interactions) in periodic systems. It was first developed as the method for calculating electrostatic energies of ionic crystals, an ...
(
), particle–particle-particle–mesh (
P3M
Particle–Particle–Particle–Mesh (P3M) is a Fourier-based Ewald summation method to calculate potentials in N-body simulations.
The potential could be the electrostatic potential among N point charges i.e. molecular dynamics, the gravitation ...
), or good spherical cutoff methods (
).
Another factor that impacts total CPU time needed by a simulation is the size of the integration timestep. This is the time length between evaluations of the potential. The timestep must be chosen small enough to avoid
discretization errors (i.e., smaller than the period related to fastest vibrational frequency in the system). Typical timesteps for classical MD are in the order of 1 femtosecond (10
−15 s). This value may be extended by using algorithms such as the SHAKE
constraint algorithm
In computational chemistry, a constraint algorithm is a method for satisfying the Newtonian motion of a rigid body which consists of mass points. A restraint algorithm is used to ensure that the distance between mass points is maintained. The gen ...
, which fix the vibrations of the fastest atoms (e.g., hydrogens) into place. Multiple time scale methods have also been developed, which allow extended times between updates of slower long-range forces.
For simulating molecules in a solvent, a choice should be made between
explicit
Explicit refers to something that is specific, clear, or detailed. It can also mean:
* Explicit knowledge, knowledge that can be readily articulated, codified and transmitted to others
* Explicit (text) The explicit (from Latin ''explicitus est'', ...
and
implicit solvent Implicit solvation (sometimes termed continuum solvation) is a method to represent solvent as a continuous medium instead of individual “explicit” solvent molecules, most often used in molecular dynamics simulations and in other applications of ...
. Explicit solvent particles (such as the
TIP3P
In computational chemistry, a water model is used to simulate and thermodynamically calculate water clusters, liquid water, and aqueous solutions with explicit solvent. The models are determined from quantum mechanics, molecular mechanics, experim ...
, SPC/E and
SPC-f water models) must be calculated expensively by the force field, while implicit solvents use a mean-field approach. Using an explicit solvent is computationally expensive, requiring inclusion of roughly ten times more particles in the simulation. But the granularity and viscosity of explicit solvent is essential to reproduce certain properties of the solute molecules. This is especially important to reproduce
chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in ...
.
In all kinds of molecular dynamics simulations, the simulation box size must be large enough to avoid
boundary condition
In mathematics, in the field of differential equations, a boundary value problem is a differential equation together with a set of additional constraints, called the boundary conditions. A solution to a boundary value problem is a solution to th ...
artifacts. Boundary conditions are often treated by choosing fixed values at the edges (which may cause artifacts), or by employing
periodic boundary conditions in which one side of the simulation loops back to the opposite side, mimicking a bulk phase (which may cause artifacts too).

Microcanonical ensemble (NVE)
In the
microcanonical ensemble
In statistical mechanics, the microcanonical ensemble is a statistical ensemble that represents the possible states of a mechanical system whose total energy is exactly specified. The system is assumed to be isolated in the sense that it canno ...
, the system is isolated from changes in moles (N), volume (V), and energy (E). It corresponds to an
adiabatic process
In thermodynamics, an adiabatic process (Greek: ''adiábatos'', "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal proces ...
with no heat exchange. A microcanonical molecular dynamics trajectory may be seen as an exchange of potential and kinetic energy, with total energy being conserved. For a system of N particles with coordinates
and velocities
, the following pair of first order differential equations may be written in
Newton's notation
In differential calculus, there is no single uniform notation for differentiation. Instead, various notations for the derivative of a function or variable have been proposed by various mathematicians. The usefulness of each notation varies with ...
as
:
:
The potential energy function
of the system is a function of the particle coordinates
. It is referred to simply as the ''potential'' in physics, or the ''force field'' in chemistry. The first equation comes from
Newton's laws of motion
Newton's laws of motion are three basic laws of classical mechanics that describe the relationship between the motion of an object and the forces acting on it. These laws can be paraphrased as follows:
# A body remains at rest, or in moti ...
; the force
acting on each particle in the system can be calculated as the negative gradient of
.
For every time step, each particle's position
and velocity
may be integrated with a
symplectic integrator In mathematics, a symplectic integrator (SI) is a numerical integration scheme for Hamiltonian systems. Symplectic integrators form the subclass of geometric integrators which, by definition, are canonical transformations. They are widely used in ...
method such as
Verlet integration
Verlet integration () is a numerical method used to integrate Newton's equations of motion. It is frequently used to calculate trajectories of particles in molecular dynamics simulations and computer graphics. The algorithm was first used in 1791 ...
. The time evolution of
and
is called a trajectory. Given the initial positions (e.g., from theoretical knowledge) and velocities (e.g., randomized Gaussian), we can calculate all future (or past) positions and velocities.
One frequent source of confusion is the meaning of
temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
in MD. Commonly we have experience with macroscopic temperatures, which involve a huge number of particles. But temperature is a statistical quantity. If there is a large enough number of atoms, statistical temperature can be estimated from the ''instantaneous temperature'', which is found by equating the kinetic energy of the system to ''nk
BT''/2 where n is the number of degrees of freedom of the system.
A temperature-related phenomenon arises due to the small number of atoms that are used in MD simulations. For example, consider simulating the growth of a copper film starting with a substrate containing 500 atoms and a deposition energy of 100 eV. In the real world, the 100 eV from the deposited atom would rapidly be transported through and shared among a large number of atoms (
or more) with no big change in temperature. When there are only 500 atoms, however, the substrate is almost immediately vaporized by the deposition. Something similar happens in biophysical simulations. The temperature of the system in NVE is naturally raised when macromolecules such as proteins undergo exothermic conformational changes and binding.
Canonical ensemble (NVT)
In the
canonical ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the heat ...
, amount of substance (N), volume (V) and temperature (T) are conserved. It is also sometimes called constant temperature molecular dynamics (CTMD). In NVT, the energy of endothermic and exothermic processes is exchanged with a thermostat.
A variety of thermostat algorithms are available to add and remove energy from the boundaries of an MD simulation in a more or less realistic way, approximating the
canonical ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the heat ...
. Popular methods to control temperature include velocity rescaling, the
Nosé–Hoover thermostat The Nosé–Hoover thermostat is a deterministic algorithm for constant-temperature molecular dynamics simulations.
It was originally developed by Nosé and was improved further by Hoover. Although the heat bath of Nosé–Hoover thermostat consis ...
, Nosé–Hoover chains, the
Berendsen thermostat The Berendsen thermostat is an algorithm to re-scale the velocities of particles in molecular dynamics simulations to control the simulation temperature.
Basic description
In this scheme, the system is weakly coupled to a heat bath with some temper ...
, the
Andersen thermostat The Andersen thermostat is a proposal in molecular dynamics simulation for maintaining constant temperature conditions. It is based on the reassignment of a chosen atom or molecule's velocity. The new velocity is given by Maxwell–Boltzmann statis ...
and
Langevin dynamics
In physics, Langevin dynamics is an approach to the mathematical modeling of the dynamics of molecular systems. It was originally developed by French physicist Paul Langevin. The approach is characterized by the use of simplified models while acco ...
. The Berendsen thermostat might introduce the
flying ice cube
In molecular dynamics (MD) simulations, the flying ice cube effect is an artifact in which the energy of high-frequency fundamental modes is drained into low-frequency modes, particularly into zero-frequency motions such as overall translation and ...
effect, which leads to unphysical translations and rotations of the simulated system.
It is not trivial to obtain a
canonical ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the heat ...
distribution of conformations and velocities using these algorithms. How this depends on system size, thermostat choice, thermostat parameters, time step and integrator is the subject of many articles in the field.
Isothermal–isobaric (NPT) ensemble
In the
isothermal–isobaric ensemble
The isothermal–isobaric ensemble (constant temperature and constant pressure ensemble) is a statistical mechanical ensemble that maintains constant temperature T \, and constant pressure P \, applied. It is also called the NpT-ensemble, where ...
, amount of substance (N), pressure (P) and temperature (T) are conserved. In addition to a thermostat, a barostat is needed. It corresponds most closely to laboratory conditions with a flask open to ambient temperature and pressure.
In the simulation of biological membranes,
isotropic pressure control is not appropriate. For lipid bilayers, pressure control occurs under constant membrane area (NPAT) or constant surface tension "gamma" (NPγT).
Generalized ensembles
The
replica exchange
Parallel tempering in physics and statistics, is a computer simulation method typically used to find the lowest free energy state of a system of many interacting particles at low temperature. That is, the one expected to be observed in reality. ...
method is a generalized ensemble. It was originally created to deal with the slow dynamics of disordered spin systems. It is also called parallel tempering. The replica exchange MD (REMD) formulation tries to overcome the multiple-minima problem by exchanging the temperature of non-interacting replicas of the system running at several temperatures.
Potentials in MD simulations
A molecular dynamics simulation requires the definition of a
potential function, or a description of the terms by which the particles in the simulation will interact. In chemistry and biology this is usually referred to as a
force field and in materials physics as an
interatomic potential
Interatomic potentials are mathematical functions to calculate the potential energy of a system of atoms with given positions in space.M. P. Allen and D. J. Tildesley. Computer Simulation of Liquids. Oxford University Press, Oxford, England, 198 ...
. Potentials may be defined at many levels of physical accuracy; those most commonly used in chemistry are based on
molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
and embody a
classical mechanics
Classical mechanics is a physical theory describing the motion of macroscopic objects, from projectiles to parts of machinery, and astronomical objects, such as spacecraft, planets, stars, and galaxies. For objects governed by classical ...
treatment of particle-particle interactions that can reproduce structural and
conformational changes but usually cannot reproduce
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
s.
The reduction from a fully quantum description to a classical potential entails two main approximations. The first one is the
Born–Oppenheimer approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and elect ...
, which states that the dynamics of electrons are so fast that they can be considered to react instantaneously to the motion of their nuclei. As a consequence, they may be treated separately. The second one treats the nuclei, which are much heavier than electrons, as point particles that follow classical Newtonian dynamics. In classical molecular dynamics, the effect of the electrons is approximated as one potential energy surface, usually representing the ground state.
When finer levels of detail are needed, potentials based on
quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
are used; some methods attempt to create hybrid
classical/quantum potentials where the bulk of the system is treated classically but a small region is treated as a quantum system, usually undergoing a chemical transformation.
Empirical potentials
Empirical potentials used in chemistry are frequently called
force fields, while those used in materials physics are called
interatomic potential
Interatomic potentials are mathematical functions to calculate the potential energy of a system of atoms with given positions in space.M. P. Allen and D. J. Tildesley. Computer Simulation of Liquids. Oxford University Press, Oxford, England, 198 ...
s.
Most
force fields in chemistry are empirical and consist of a summation of bonded forces associated with
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s, bond angles, and bond
dihedrals, and non-bonded forces associated with
van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s and
electrostatic charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectiv ...
. Empirical potentials represent quantum-mechanical effects in a limited way through ad hoc functional approximations. These potentials contain free parameters such as
atomic charge, van der Waals parameters reflecting estimates of
atomic radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
, and equilibrium
bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
, angle, and dihedral; these are obtained by fitting against detailed electronic calculations (quantum chemical simulations) or experimental physical properties such as
elastic constants, lattice parameters and
spectroscopic
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter wa ...
measurements.
Because of the non-local nature of non-bonded interactions, they involve at least weak interactions between all particles in the system. Its calculation is normally the bottleneck in the speed of MD simulations. To lower the computational cost,
force fields employ numerical approximations such as shifted cutoff radii,
reaction field algorithms, particle mesh
Ewald summation Ewald summation, named after Paul Peter Ewald, is a method for computing long-range interactions (e.g. electrostatic interactions) in periodic systems. It was first developed as the method for calculating electrostatic energies of ionic crystals, an ...
, or the newer particle–particle-particle–mesh (
P3M
Particle–Particle–Particle–Mesh (P3M) is a Fourier-based Ewald summation method to calculate potentials in N-body simulations.
The potential could be the electrostatic potential among N point charges i.e. molecular dynamics, the gravitation ...
).
Chemistry force fields commonly employ preset bonding arrangements (an exception being ''
ab initio
''Ab initio'' ( ) is a Latin term meaning "from the beginning" and is derived from the Latin ''ab'' ("from") + ''initio'', ablative singular of ''initium'' ("beginning").
Etymology
Circa 1600, from Latin, literally "from the beginning", from ab ...
'' dynamics), and thus are unable to model the process of chemical bond breaking and reactions explicitly. On the other hand, many of the potentials used in physics, such as those based on the
bond order formalism can describe several different coordinations of a system and bond breaking. Examples of such potentials include the
Brenner potential
Bond order potential is a class of empirical (analytical) interatomic potentials which is used in molecular dynamics and molecular statics simulations. Examples include the Tersoff potential, the EDIP potential, the Brenner potential, the Finnis� ...
for hydrocarbons and its
further developments for the C-Si-H and C-O-H systems. The
ReaxFF ReaxFF (for “reactive force field”) is a bond order-based force field developed by Adri van Duin, William A. Goddard, III, and co-workers at the California Institute of Technology. One of its applications is molecular dynamics
Molecular ...
potential can be considered a fully reactive hybrid between bond order potentials and chemistry force fields.
Pair potentials versus many-body potentials
The potential functions representing the non-bonded energy are formulated as a sum over interactions between the particles of the system. The simplest choice, employed in many popular
force fields, is the "pair potential", in which the total potential energy can be calculated from the sum of energy contributions between pairs of atoms. Therefore, these force fields are also called "additive force fields". An example of such a pair potential is the non-bonded
Lennard–Jones potential
The Lennard-Jones potential (also termed the LJ potential or 12-6 potential) is an intermolecular pair potential. Out of all the intermolecular potentials, the Lennard-Jones potential is probably the one that has been the most extensively studie ...
(also termed the 6–12 potential), used for calculating van der Waals forces.
:
Another example is the Born (ionic) model of the ionic lattice. The first term in the next equation is
Coulomb's law
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is convention ...
for a pair of ions, the second term is the short-range repulsion explained by Pauli's exclusion principle and the final term is the dispersion interaction term. Usually, a simulation only includes the dipolar term, although sometimes the quadrupolar term is also included. When ''n
l'' = 6, this potential is also called the
Coulomb–Buckingham potential.
:
In
many-body potentials, the potential energy includes the effects of three or more particles interacting with each other.
In simulations with pairwise potentials, global interactions in the system also exist, but they occur only through pairwise terms. In many-body potentials, the potential energy cannot be found by a sum over pairs of atoms, as these interactions are calculated explicitly as a combination of higher-order terms. In the statistical view, the dependency between the variables cannot in general be expressed using only pairwise products of the degrees of freedom. For example, the
Tersoff potential, which was originally used to simulate
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
,
silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic tab ...
, and
germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors s ...
, and has since been used for a wide range of other materials, involves a sum over groups of three atoms, with the angles between the atoms being an important factor in the potential. Other examples are the
embedded-atom method (EAM), the EDIP,
and the Tight-Binding Second Moment Approximation (TBSMA) potentials, where the electron density of states in the region of an atom is calculated from a sum of contributions from surrounding atoms, and the potential energy contribution is then a function of this sum.
Semi-empirical potentials
Semi-empirical
Empirical evidence for a proposition is evidence, i.e. what supports or counters this proposition, that is constituted by or accessible to sense experience or experimental procedure. Empirical evidence is of central importance to the sciences an ...
potentials make use of the matrix representation from quantum mechanics. However, the values of the matrix elements are found through empirical formulae that estimate the degree of overlap of specific atomic orbitals. The matrix is then diagonalized to determine the occupancy of the different atomic orbitals, and empirical formulae are used once again to determine the energy contributions of the orbitals.
There are a wide variety of semi-empirical potentials, termed
tight-binding
In solid-state physics, the tight-binding model (or TB model) is an approach to the calculation of electronic band structure using an approximate set of wave functions based upon superposition of wave functions for isolated atoms located at each ...
potentials, which vary according to the atoms being modeled.
Polarizable potentials
Most classical force fields implicitly include the effect of
polarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, considering that matter is made up of elementar ...
, e.g., by scaling up the partial charges obtained from quantum chemical calculations. These partial charges are stationary with respect to the mass of the atom. But molecular dynamics simulations can explicitly model polarizability with the introduction of induced dipoles through different methods, such as
Drude particle Drude particles are model oscillators used to simulate the effects of electronic polarizability in the context of a classical molecular mechanics force field. They are inspired by the Drude model of mobile electrons and are used in the computatio ...
s or fluctuating charges. This allows for a dynamic redistribution of charge between atoms which responds to the local chemical environment.
For many years, polarizable MD simulations have been touted as the next generation. For homogenous liquids such as water, increased accuracy has been achieved through the inclusion of polarizability.
Some promising results have also been achieved for proteins.
However, it is still uncertain how to best approximate polarizability in a simulation. The point becomes more important when a particle experiences different environments during its simulation trajectory, e.g. translocation of a drug through a cell membrane.
Potentials in ''ab initio'' methods
In classical molecular dynamics, one potential energy surface (usually the ground state) is represented in the force field. This is a consequence of the
Born–Oppenheimer approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and elect ...
. In excited states, chemical reactions or when a more accurate representation is needed, electronic behavior can be obtained from first principles using a quantum mechanical method, such as
density functional theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
. This is named ''Ab Initio Molecular Dynamics'' (AIMD). Due to the cost of treating the electronic degrees of freedom, the computational burden of these simulations is far higher than classical molecular dynamics. For this reason, AIMD is typically limited to smaller systems and shorter times.
''
Ab initio
''Ab initio'' ( ) is a Latin term meaning "from the beginning" and is derived from the Latin ''ab'' ("from") + ''initio'', ablative singular of ''initium'' ("beginning").
Etymology
Circa 1600, from Latin, literally "from the beginning", from ab ...
''
quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
and
chemical
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
methods may be used to calculate the
potential energy
In physics, potential energy is the energy held by an object because of its position relative to other objects, stresses within itself, its electric charge, or other factors.
Common types of potential energy include the gravitational potentia ...
of a system on the fly, as needed for conformations in a trajectory. This calculation is usually made in the close neighborhood of the
reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities. In molecu ...
. Although various approximations may be used, these are based on theoretical considerations, not on empirical fitting. ''Ab initio'' calculations produce a vast amount of information that is not available from empirical methods, such as density of electronic states or other electronic properties. A significant advantage of using ''ab initio'' methods is the ability to study reactions that involve breaking or formation of covalent bonds, which correspond to multiple electronic states. Moreover, ''ab initio'' methods also allow recovering effects beyond the
Born–Oppenheimer approximation
In quantum chemistry and molecular physics, the Born–Oppenheimer (BO) approximation is the best-known mathematical approximation in molecular dynamics. Specifically, it is the assumption that the wave functions of atomic nuclei and elect ...
using approaches like
mixed quantum-classical dynamics
Mixed quantum-classical (MQC) dynamics is a class of computational theoretical chemistry methods tailored to simulate non- adiabatic (NA) processes in molecular and supramolecular chemistry. Such methods are characterized by:
# Propagation of n ...
.
Hybrid QM/MM
QM (quantum-mechanical) methods are very powerful. However, they are computationally expensive, while the MM (classical or molecular mechanics) methods are fast but suffer from several limits (require extensive parameterization; energy estimates obtained are not very accurate; cannot be used to simulate reactions where covalent bonds are broken/formed; and are limited in their abilities for providing accurate details regarding the chemical environment). A new class of method has emerged that combines the good points of QM (accuracy) and MM (speed) calculations. These methods are termed mixed or hybrid quantum-mechanical and molecular mechanics methods (hybrid QM/MM).
The most important advantage of hybrid QM/MM method is the speed. The cost of doing classical molecular dynamics (MM) in the most straightforward case scales O(n
2), where n is the number of atoms in the system. This is mainly due to electrostatic interactions term (every particle interacts with every other particle). However, use of cutoff radius, periodic pair-list updates and more recently the variations of the particle-mesh Ewald's (PME) method has reduced this to between O(n) to O(n
2). In other words, if a system with twice as many atoms is simulated then it would take between two and four times as much computing power. On the other hand, the simplest ''ab initio'' calculations typically scale O(n
3) or worse (restricted
Hartree–Fock calculations have been suggested to scale ~O(n
2.7)). To overcome the limit, a small part of the system is treated quantum-mechanically (typically active-site of an enzyme) and the remaining system is treated classically.
In more sophisticated implementations, QM/MM methods exist to treat both light nuclei susceptible to quantum effects (such as hydrogens) and electronic states. This allows generating hydrogen wave-functions (similar to electronic wave-functions). This methodology has been useful in investigating phenomena such as hydrogen tunneling. One example where QM/MM methods have provided new discoveries is the calculation of hydride transfer in the enzyme liver
alcohol dehydrogenase
Alcohol dehydrogenases (ADH) () are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NA ...
. In this case,
quantum tunneling
In physics, a quantum (plural quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a physical property can be "quantized" is referred to as "the hypothesis of quantizati ...
is important for the hydrogen, as it determines the reaction rate.
Coarse-graining and reduced representations
At the other end of the detail scale are
coarse-grained
Granularity (also called graininess), the condition of existing in granules or grains, refers to the extent to which a material or system is composed of distinguishable pieces. It can either refer to the extent to which a larger entity is sub ...
and lattice models. Instead of explicitly representing every atom of the system, one uses "pseudo-atoms" to represent groups of atoms. MD simulations on very large systems may require such large computer resources that they cannot easily be studied by traditional all-atom methods. Similarly, simulations of processes on long timescales (beyond about 1 microsecond) are prohibitively expensive, because they require so many time steps. In these cases, one can sometimes tackle the problem by using reduced representations, which are also called
coarse-grained models.
Examples for coarse graining (CG) methods are discontinuous molecular dynamics (CG-DMD) and Go-models. Coarse-graining is done sometimes taking larger pseudo-atoms. Such united atom approximations have been used in MD simulations of biological membranes. Implementation of such approach on systems where electrical properties are of interest can be challenging owing to the difficulty of using a proper charge distribution on the pseudo-atoms. The aliphatic tails of lipids are represented by a few pseudo-atoms by gathering 2 to 4 methylene groups into each pseudo-atom.
The parameterization of these very
coarse-grained models must be done empirically, by matching the behavior of the model to appropriate experimental data or all-atom simulations. Ideally, these parameters should account for both
enthalpic
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
and
entropic
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
contributions to free energy in an implicit way. When coarse-graining is done at higher levels, the accuracy of the dynamic description may be less reliable. But very
coarse-grained models have been used successfully to examine a wide range of questions in structural biology, liquid crystal organization, and polymer glasses.
Examples of applications of coarse-graining:
*
protein folding
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduc ...
and
protein structure prediction
Protein structure prediction is the inference of the three-dimensional structure of a protein from its amino acid sequence—that is, the prediction of its secondary and tertiary structure from primary structure. Structure prediction is different ...
studies are often carried out using one, or a few, pseudo-atoms per amino acid;
*
liquid crystal phase transitions have been examined in confined geometries and/or during flow using the
Gay-Berne potential, which describes anisotropic species;
*
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
glasses during deformation have been studied using simple harmonic or
FENE
Fene is a municipality in the province of A Coruña in the autonomous community of Galicia in northwestern Spain. It is located to the northeast of Galicia on the Ria of Ferrol.
Economy
The Navantia Shipyards and services in the parts which ...
springs to connect spheres described by the
Lennard-Jones potential;
*
DNA supercoiling
DNA supercoiling refers to the amount of twist in a particular DNA strand, which determines the amount of strain on it. A given strand may be "positively supercoiled" or "negatively supercoiled" (more or less tightly wound). The amount of a st ...
has been investigated using 1–3 pseudo-atoms per basepair, and at even lower resolution;
* Packaging of
double-helical DNA into
bacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteri ...
has been investigated with models where one pseudo-atom represents one turn (about 10 basepairs) of the double helix;
* RNA structure in the
ribosome
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to ...
and other large systems has been modeled with one pseudo-atom per nucleotide.
* Virtual cell simulation to study the interaction of cells and various substrates.
The simplest form of coarse-graining is the ''united atom'' (sometimes called ''extended atom'') and was used in most early MD simulations of proteins, lipids, and nucleic acids. For example, instead of treating all four atoms of a CH
3 methyl group explicitly (or all three atoms of CH
2 methylene group), one represents the whole group with one pseudo-atom. It must, of course, be properly parameterized so that its van der Waals interactions with other groups have the proper distance-dependence. Similar considerations apply to the bonds, angles, and torsions in which the pseudo-atom participates. In this kind of united atom representation, one typically eliminates all explicit hydrogen atoms except those that have the capability to participate in hydrogen bonds (''polar hydrogens''). An example of this is the
CHARMM
Chemistry at Harvard Macromolecular Mechanics (CHARMM) is the name of a widely used set of force fields for molecular dynamics, and the name for the molecular dynamics simulation and analysis computer software package associated with them. The CHA ...
19 force-field.
The polar hydrogens are usually retained in the model, because proper treatment of hydrogen bonds requires a reasonably accurate description of the directionality and the electrostatic interactions between the donor and acceptor groups. A hydroxyl group, for example, can be both a hydrogen bond donor, and a hydrogen bond acceptor, and it would be impossible to treat this with one OH pseudo-atom. About half the atoms in a protein or nucleic acid are non-polar hydrogens, so the use of united atoms can provide a substantial savings in computer time.
Incorporating solvent effects
In many simulations of a solute-solvent system the main focus is on the behavior of the solute with little interest of the solvent behavior particularly in those solvent molecules residing in regions far from the solute molecule. Solvents may influence the dynamic behavior of solutes via random collisions and by imposing a frictional drag on the motion of the solute through the solvent. The use of non-rectangular periodic boundary conditions, stochastic boundaries and solvent shells can all help reduce the number of solvent molecules required and enable a larger proportion of the computing time to be spent instead on simulating the solute. It is also possible to incorporate the effects of a solvent without needing any explicit solvent molecules present. One example of this approach is to use a
potential mean force (PMF) which describes how the free energy changes as a particular coordinate is varied. The free energy change described by PMF contains the averaged effects of the solvent.
Without incorporating the effects of solvent simulations of macromolecules (such as proteins) may yield unrealistic behavior and even small molecules may adopt more compact conformations due to favourable van der Waals forces and electrostatic interactions which would be dampened in the presence of a solvent.
Long-range forces
A long range interaction is an interaction in which the spatial interaction falls off no faster than
where
is the dimensionality of the system. Examples include charge-charge interactions between ions and dipole-dipole interactions between molecules. Modelling these forces presents quite a challenge as they are significant over a distance which may be larger than half the box length with simulations of many thousands of particles. Though one solution would be to significantly increase the size of the box length, this brute force approach is less than ideal as the simulation would become computationally very expensive. Spherically truncating the potential is also out of the question as unrealistic behaviour may be observed when the distance is close to the cut off distance.
Steered molecular dynamics (SMD)
Steered molecular dynamics (SMD) simulations, or force probe simulations, apply forces to a protein in order to manipulate its structure by pulling it along desired degrees of freedom. These experiments can be used to reveal structural changes in a protein at the atomic level. SMD is often used to simulate events such as mechanical unfolding or stretching.
There are two typical protocols of SMD: one in which pulling velocity is held constant, and one in which applied force is constant. Typically, part of the studied system (e.g., an atom in a protein) is restrained by a harmonic potential. Forces are then applied to specific atoms at either a constant velocity or a constant force.
Umbrella sampling
Umbrella sampling is a technique in computational physics and chemistry, used to improve sampling of a system (or different systems) where ergodicity is hindered by the form of the system's energy landscape. It was first suggested by Torrie and ...
is used to move the system along the desired reaction coordinate by varying, for example, the forces, distances, and angles manipulated in the simulation. Through umbrella sampling, all of the system's configurations—both high-energy and low-energy—are adequately sampled. Then, each configuration's change in free energy can be calculated as the
potential of mean force When examining a system computationally one may be interested in knowing how the free energy changes as a function of some inter- or intramolecular coordinate (such as the distance between two atoms or a torsional angle). The free energy surface alo ...
.
A popular method of computing PMF is through the weighted histogram analysis method (WHAM), which analyzes a series of umbrella sampling simulations.
A lot of important applications of SMD are in the field of drug discovery and biomolecular sciences. For e.g. SMD was used to investigate the stability of Alzheimer's protofibrils, to study the protein ligand interaction in cyclin-dependent kinase 5 and even to show the effect of electric field on thrombin (protein) and aptamer (nucleotide) complex among many other interesting studies.
Examples of applications

Molecular dynamics is used in many fields of science.
* First MD simulation of a simplified biological folding process was published in 1975. Its simulation published in Nature paved the way for the vast area of modern computational protein-folding.
* First MD simulation of a biological process was published in 1976. Its simulation published in Nature paved the way for understanding protein motion as essential in function and not just accessory.
* MD is the standard method to treat
collision cascade
In condensed-matter physics, a collision cascade (also known as a displacement cascade or a displacement spike) is a set of nearby adjacent energetic (much higher than ordinary thermal energies) collisions of atoms induced by an energetic par ...
s in the heat spike regime, i.e., the effects that energetic
neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons beh ...
and
ion irradiation have on solids and solid surfaces.
The following biophysical examples illustrate notable efforts to produce simulations of a systems of very large size (a complete virus) or very long simulation times (up to 1.112 milliseconds):
* MD simulation of the full ''
satellite tobacco mosaic virus
''Tobacco virtovirus 1'', informally called Tobacco mosaic satellite virus, Satellite tobacco mosaic virus (STMV), or tobacco mosaic satellite virus, is a satellite virus first reported in ''Nicotiana glauca'' from southern California, U.S.. Its ...
'' (STMV) (2006, Size: 1 million atoms, Simulation time: 50 ns, program:
NAMD
Nanoscale Molecular Dynamics (NAMD, formerly Not Another Molecular Dynamics Program) is computer software for molecular dynamics simulation, written using the Charm++ parallel programming model. It is noted for its parallel efficiency and is often ...
) This virus is a small, icosahedral plant virus that worsens the symptoms of infection by Tobacco Mosaic Virus (TMV). Molecular dynamics simulations were used to probe the mechanisms of
viral assembly. The entire STMV particle consists of 60 identical copies of one protein that make up the viral
capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or ma ...
(coating), and a 1063 nucleotide single stranded RNA
genome
In the fields of molecular biology and genetics, a genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding ge ...
. One key finding is that the capsid is very unstable when there is no RNA inside. The simulation would take one 2006 desktop computer around 35 years to complete. It was thus done in many processors in parallel with continuous communication between them.
* Folding simulations of the
Villin
Villin-1 is a 92.5 kDa tissue-specific actin-binding protein associated with the actin core bundle of the brush border. Villin-1 is encoded by the ''VIL1'' gene. Villin-1 contains multiple gelsolin-like domains capped by a small (8.5 kDa) "headp ...
Headpiece in all-atom detail (2006, Size: 20,000 atoms; Simulation time: 500 μs= 500,000 ns, Program:
Folding@home
Folding@home (FAH or F@h) is a volunteer computing project aimed to help scientists develop new therapeutics for a variety of diseases by the means of simulating protein dynamics. This includes the process of protein folding and the movements ...
) This simulation was run in 200,000 CPU's of participating personal computers around the world. These computers had the Folding@home program installed, a large-scale distributed computing effort coordinated by
Vijay Pande
Vijay Satyanand Pande is a Trinidadian-American venture capitalist. Pande is the former director of the biophysics program and is best known for orchestrating the distributed computing disease research project known as Folding@home. His research ...
at Stanford University. The kinetic properties of the Villin Headpiece protein were probed by using many independent, short trajectories run by CPU's without continuous real-time communication. One method employed was the Pfold value analysis, which measures the probability of folding before unfolding of a specific starting conformation. Pfold gives information about
transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
structures and an ordering of conformations along the
folding pathway. Each trajectory in a Pfold calculation can be relatively short, but many independent trajectories are needed.
* Long continuous-trajectory simulations have been performed on
Anton
Anton may refer to: People
*Anton (given name), including a list of people with the given name
*Anton (surname)
Places
*Anton Municipality, Bulgaria
**Anton, Sofia Province, a village
*Antón District, Panama
**Antón, a town and capital of th ...
, a massively parallel supercomputer designed and built around custom
application-specific integrated circuit
An application-specific integrated circuit (ASIC ) is an integrated circuit (IC) chip customized for a particular use, rather than intended for general-purpose use, such as a chip designed to run in a digital voice recorder or a high-effici ...
s (ASICs) and interconnects by
D. E. Shaw Research. The longest published result of a simulation performed using Anton is a 1.112-millisecond simulation of NTL9 at 355 K; a second, independent 1.073-millisecond simulation of this configuration was also performed (and many other simulations of over 250 μs continuous chemical time).
In ''How Fast-Folding Proteins Fold'', researchers Kresten Lindorff-Larsen, Stefano Piana, Ron O. Dror, and
David E. Shaw
David Elliot Shaw (born March 29, 1951) is an American billionaire scientist and former hedge fund manager. He founded D. E. Shaw & Co., a hedge fund company which was once described by '' Fortune'' magazine as "the most intriguing and mysterio ...
discuss "the results of atomic-level molecular dynamics simulations, over periods ranging between 100 μs and 1 ms, that reveal a set of common principles underlying the folding of 12 structurally diverse proteins." Examination of these diverse long trajectories, enabled by specialized, custom hardware, allow them to conclude that "In most cases, folding follows a single dominant route in which elements of the native structure appear in an order highly correlated with their propensity to form in the unfolded state."
In a separate study, Anton was used to conduct a 1.013-millisecond simulation of the native-state dynamics of bovine pancreatic trypsin inhibitor (BPTI) at 300 K.
Another important application of MD method benefits from its ability of 3-dimensional characterization and analysis of microstructural evolution at atomic scale.
* MD simulations are used in characterization of grain size evolution, for example, when describing wear and friction of nanocrystalline Al and Al(Zr) materials. Dislocations evolution and grain size evolution are analyzed during the friction process in this simulation. Since MD method provided the full information of the microstructure, the grain size evolution was calculated in 3D using the Polyhedral Template Matching, Grain Segmentation, and Graph clustering
methods. In such simulation, MD method provided an accurate measurement of grain size. Making use of these information, the actual grain structures were extracted, measured, and presented. Compared to the traditional method of using SEM with a single 2-dimensional slice of the material, MD provides a 3-dimensional and accurate way to characterize the microstructural evolution at atomic scale.
Molecular dynamics algorithms
*
Screened Coulomb potentials implicit solvent model
Integrators
*
Symplectic integrator In mathematics, a symplectic integrator (SI) is a numerical integration scheme for Hamiltonian systems. Symplectic integrators form the subclass of geometric integrators which, by definition, are canonical transformations. They are widely used in ...
*
Verlet–Stoermer integration
*
Runge–Kutta integration
*
Beeman's algorithm
Beeman's algorithm is a method for numerically integrating ordinary differential equations of order 2, more specifically Newton's equations of motion \ddot x=A(x). It was designed to allow high numbers of particles in simulations of molecular dyna ...
*
Constraint algorithm
In computational chemistry, a constraint algorithm is a method for satisfying the Newtonian motion of a rigid body which consists of mass points. A restraint algorithm is used to ensure that the distance between mass points is maintained. The gen ...
s (for constrained systems)
Short-range interaction algorithms
*
Cell lists Cell lists (also sometimes referred to as cell linked-lists) is a data structure in molecular dynamics simulations to find all atom pairs within a given cut-off distance of each other. These pairs are needed to compute the short-range non-bonded int ...
*
Verlet list A Verlet list (named after Loup Verlet) is a data structure in molecular dynamics simulations to efficiently maintain a list of all particles within a given cut-off distance of each other.
This method may easily be applied to Monte Carlo simulation ...
* Bonded interactions
Long-range interaction algorithms
*
Ewald summation Ewald summation, named after Paul Peter Ewald, is a method for computing long-range interactions (e.g. electrostatic interactions) in periodic systems. It was first developed as the method for calculating electrostatic energies of ionic crystals, an ...
* Particle mesh
Ewald summation Ewald summation, named after Paul Peter Ewald, is a method for computing long-range interactions (e.g. electrostatic interactions) in periodic systems. It was first developed as the method for calculating electrostatic energies of ionic crystals, an ...
(PME)
* Particle–particle-particle–mesh (
P3M
Particle–Particle–Particle–Mesh (P3M) is a Fourier-based Ewald summation method to calculate potentials in N-body simulations.
The potential could be the electrostatic potential among N point charges i.e. molecular dynamics, the gravitation ...
)
*
Shifted force method The net electrostatic force acting on a charged particle with index i contained within a collection of particles is given as:
\mathbf(\mathbf) = \sum_F(r)\mathbf \, \, \,; \, \, F(r) = \frac
where \mathbf is the spatial coordinate, j is a partic ...
Parallelization strategies
*
Domain decomposition method
In mathematics, numerical analysis, and numerical partial differential equations, domain decomposition methods solve a boundary value problem by splitting it into smaller boundary value problems on subdomains and iterating to coordinate the solu ...
(Distribution of system data for
parallel computing
Parallel computing is a type of computation in which many calculations or processes are carried out simultaneously. Large problems can often be divided into smaller ones, which can then be solved at the same time. There are several different fo ...
)
Ab-initio molecular dynamics
*
Car–Parrinello molecular dynamics Car–Parrinello molecular dynamics or CPMD refers to either a method used in molecular dynamics (also known as the Car–Parrinello method) or the computational chemistry software package used to implement this method.
The CPMD method is one of th ...
Specialized hardware for MD simulations
*
Anton
Anton may refer to: People
*Anton (given name), including a list of people with the given name
*Anton (surname)
Places
*Anton Municipality, Bulgaria
**Anton, Sofia Province, a village
*Antón District, Panama
**Antón, a town and capital of th ...
– A specialized, massively parallel supercomputer designed to execute MD simulations
*
MDGRAPE
MDGRAPE-3 is an ultra-high performance petascale supercomputer system developed by the Riken research institute in Japan. It is a special purpose system built for molecular dynamics simulations, especially protein structure prediction.
MDGRAPE- ...
– A special purpose system built for molecular dynamics simulations, especially protein structure prediction
Graphics card as a hardware for MD simulations
See also
*
Molecular modeling
Molecular modelling encompasses all methods, theoretical and computational, used to model or mimic the behaviour of molecules. The methods are used in the fields of computational chemistry, drug design, computational biology and materials scien ...
*
Computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of m ...
*
Force field (chemistry)
In the context of chemistry and molecular modelling, a force field is a computational method that is used to estimate the forces between atoms within molecules and also between molecules. More precisely, the force field refers to the function ...
*
Comparison of force field implementations
This is a table of notable computer programs implementing molecular mechanics force fields.
See also
*Force field (chemistry)
* List of software for Monte Carlo molecular modeling
*Molecular mechanics
* Molecular design software
*Molecule edi ...
*
Monte Carlo method
Monte Carlo methods, or Monte Carlo experiments, are a broad class of computational algorithms that rely on repeated random sampling to obtain numerical results. The underlying concept is to use randomness to solve problems that might be determi ...
*
Molecular design software Molecular design software is notable software for molecular modeling, that provides special support for developing molecular models ''de novo''.
In contrast to the usual molecular modeling programs, such as for molecular dynamics and quantum chemi ...
*
Molecular mechanics
Molecular mechanics uses classical mechanics to model molecular systems. The Born–Oppenheimer approximation is assumed valid and the potential energy of all systems is calculated as a function of the nuclear coordinates using Force field (chemi ...
*
Multiscale Green's function
Multiscale Green's function (MSGF) is a generalized and extended version of the classical Green's function (GF) technique for solving mathematical equations. The main application of the MSGF technique is in modeling of nanomaterials. These materi ...
*
Car–Parrinello method
*
Comparison of software for molecular mechanics modeling
This is a list of computer programs that are predominantly used for molecular mechanics calculations.
See also
* Car–Parrinello molecular dynamics
* Comparison of force-field implementations
*Comparison of nucleic acid simulation software ...
*
Quantum chemistry
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions ...
*
Discrete element method
A discrete element method (DEM), also called a distinct element method, is any of a family of numerical methods for computing the motion and effect of a large number of small particles. Though DEM is very closely related to molecular dynamics, t ...
*
Comparison of nucleic acid simulation software
This is a list of notable computer programs that are used for nucleic acids simulations.
See also
References
Computational chemistry software
Software comparisons
Molecular dynamics software
Molecular modelling software
{{sc ...
*
Molecule editor
A molecule editor is a computer program for creating and modifying representations of chemical structures.
Molecule editors can manipulate chemical structure representations in either a simulated two-dimensional space or three-dimensional space, v ...
*
Mixed quantum-classical dynamics
Mixed quantum-classical (MQC) dynamics is a class of computational theoretical chemistry methods tailored to simulate non- adiabatic (NA) processes in molecular and supramolecular chemistry. Such methods are characterized by:
# Propagation of n ...
References
General references
*
*
*
*
*
*
*
*
*
*
*
*
External links
{{Commons category, Molecular dynamics simulation
The GPUGRID.net Project(
GPUGRID.net
GPUGRID is a volunteer computing project hosted by Pompeu Fabra University and running on the Berkeley Open Infrastructure for Network Computing (BOINC) software platform. It performs full-atom molecular biology simulations that are designed t ...
)
The Blue Gene Project(
IBM)JawBreakers.org
Materials modelling and computer simulation codesA few tips on molecular dynamicsMovie of MD simulation of water (Youtube)
Computational chemistry
Molecular modelling
Simulation
 Molecular dynamics (MD) is a
Molecular dynamics (MD) is a  In 1957,
In 1957, 
 Molecular dynamics is used in many fields of science.
* First MD simulation of a simplified biological folding process was published in 1975. Its simulation published in Nature paved the way for the vast area of modern computational protein-folding.
* First MD simulation of a biological process was published in 1976. Its simulation published in Nature paved the way for understanding protein motion as essential in function and not just accessory.
* MD is the standard method to treat
Molecular dynamics is used in many fields of science.
* First MD simulation of a simplified biological folding process was published in 1975. Its simulation published in Nature paved the way for the vast area of modern computational protein-folding.
* First MD simulation of a biological process was published in 1976. Its simulation published in Nature paved the way for understanding protein motion as essential in function and not just accessory.
* MD is the standard method to treat